The US Food and Drug Administration has given marketing clearance to a blood test to help diagnose Alzheimer’s disease, making the test the first to get signoff to aid in the early detection of the disease in the United States.
The test, called the Lumipulse G pTau217/ß-Amyloid 1-42 Plasma Ratio, is for adults 55 and older who are showing signs and symptoms of Alzheimer’s disease, the FDA announced Friday.
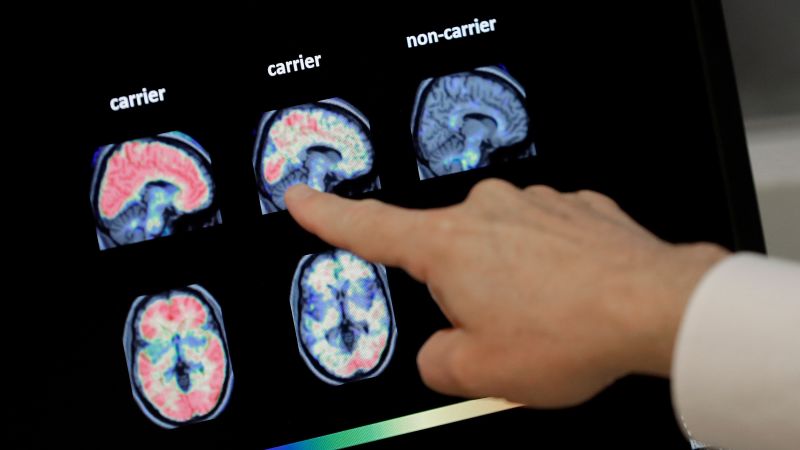
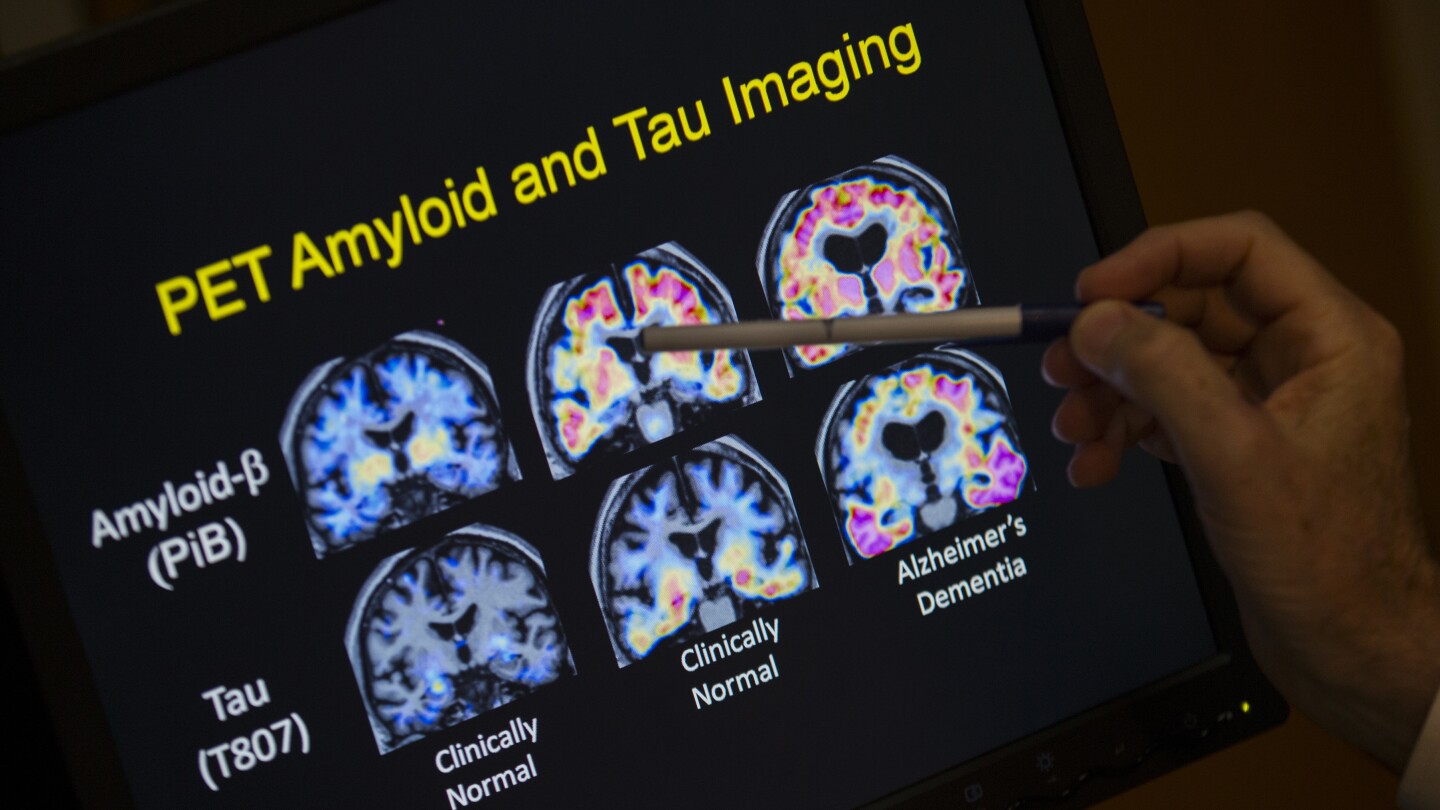
It works by measuring two proteins in blood plasma: pTau217 and beta-amyloid 1-42. A ratio of those proteins tends to correlate with the occurrence or absence of amyloid plaques in the brain, which are among the hallmarks of Alzheimer’s disease. The test does not measure amyloid directly but can signal its presence.
However, there remains no current single test to diagnose Alzheimer’s disease. Doctors primarily rely on a variety of tools to diagnose the condition, which may include medical history, neurological exams, cognitive and functional evaluations, brain imaging, spinal fluid analysis and, more recently, blood tests.
The FDA said the results of the newly cleared blood test must be assessed in conjunction with other clinical information from a patient.
“Alzheimer’s disease impacts too many people, more than breast cancer and prostate cancer combined,” FDA Commissioner Dr. Martin Makary said in Friday’s announcement. “Knowing that 10% of people aged 65 and older have Alzheimer’s, and that by 2050 that number is expected to double, I am hopeful that new medical products such as this one will help patients.”
According to the FDA, the new blood test – developed by the Pennsylvania-based biotechnology company Fujirebio Diagnostics Inc. – can help increase access to Alzheimer’s disease detection and reduce reliance on positron emission tomography or PET scans, a type of imaging that can reveal amyloid plaques in the brain but can be expensive, costing thousands of dollars without insurance.
The FDA said it reviewed clinical trial data on the new blood test, involving plasma samples collected from 499 adults who were cognitively impaired. The samples were evaluated using the blood test, and the results were compared with the results from patients’ PET scans or separate testing using cerebrospinal fluid samples, such as from spinal taps.
The data showed that 91.7% of adults with positive results using the blood test had the presence of amyloid plaques confirmed by their PET scan or cerebrospinal fluid test, and 97.3% of people with negative results had a negative amyloid PET scan or cerebrospinal fluid test result, according to the FDA.
The agency added that the risks associated with the blood test are mainly the risk of a false positive or false negative test result.
Preventive neurologist Dr. Richard Isaacson, who established one of the first Alzheimer’s prevention clinics in the United States, said he has been using this blood test for years for research and applauded the FDA clearance.
“It can provide better clarity into whether a person experiencing memory loss may have Alzheimer’s disease. They can take this test as a screening test,” said Isaacson, director of research at the Institute for Neurodegenerative Diseases in Florida. Compared with costly PET scans or spinal taps, “this is a much more simple screening test, with reasonable accuracy, to tell the physician that a person with cognitive decline has symptoms that are actually due to Alzheimer’s disease.”
But Isaacson warned that while the FDA clearance is “an important step forward” for the field, more research is needed to help inform how blood test results should be interpreted and used to make clinical decisions.
“I think the next step as a field is, we need to advance education about what these tests mean and what they don’t and who they should be used for,” he said. “Because they mean different things in different people depending on their risk factors and whether or not they have symptoms. So we’re still early.”
Fujirebio Diagnostics designed the blood test to help detect Alzheimer’s disease early, when interventions are more effective, president and CEO Monte Wiltse said in a news release last year, when the company filed its test with the FDA.
“An early and accurate diagnosis will also facilitate the development of new drug therapies, which are urgently needed as the prevalence of AD increases with a rapidly aging population globally,” Wiltse said.
It’s estimated that more than 2 in 5 people over the age of 55 in the United States – about 42% – will develop dementia in their later years.
But in some cases, deposits of amyloid can start to accumulate in the brain decades before Alzheimer’s symptoms begin. Early detection of these amyloid plaques could open the door for a person to take steps to slow the progression of disease, such as starting preventive treatment with medications.
“For too long Americans have struggled to get a simple and accurate diagnosis, with today’s action by the FDA we are hopeful it will be easier for more individuals to receive an accurate diagnosis earlier,” Dr. Maria Carrillo, chief science officer and medical affairs lead at the Alzheimer’s Association, said in a statement Friday.
There are a variety of laboratory-developed tests on the market that can be used to detect blood-based biomarkers associated with Alzheimer’s, according to the Alzheimer’s Association, as well as experimental tests. But the Fujirebio Diagnostics test is the first one cleared by the FDA.
“Blood-based biomarkers are reshaping how we identify and understand Alzheimer’s disease,” Carrillo said. “At the same time, there are important questions for health care professionals to consider; in particular, who should be tested and when.”
For now, the FDA’s clearance “marks a major milestone,” said Dr. Howard Fillit, co-founder and chief science officer at the Alzheimer’s Drug Discovery Foundation.
“The ability to diagnose Alzheimer’s earlier with a simple blood test, like we do for cholesterol, is a game changer, allowing more patients to receive treatment options that have the potential to significantly slow or even prevent the disease,” Fillit said in an email Friday. “This is a clear example of the new era of Alzheimer’s research where innovation, science and technology come together to develop more accessible, affordable and scalable tools that will pave the way for additional regulatory approvals of diagnostic tools.”