Alzheimer’s numbers expected to hit 13 million by 2050
Dr. Joanne Pike, CEO of the Alzheimer’s Association, talks about the challenges and hopes when it comes to this debilitating disease.
We’re now closer to being able to diagnose most common form of dementia with a simple test. The Food and Drug Administration on May 16 said it cleared the first blood test for Alzheimer’s disease.
The blood test is for patients 55 and older who have signs and symptoms of the mind-robbing disease.
The Lumipulse blood plasma test detects a disease hallmark, amyloid plaques, which form in the brains of Alzheimer’s patients.
This approval marks a milestone for patients, their families and doctors, said Howard Fillit, co-founder and chief science officer at the Alzheimer’s Drug Discovery Foundation.
“The ability to diagnose Alzheimer’s earlier with a simple blood test, like we do for cholesterol, is a game changer, allowing more patients to receive treatment options that have the potential to significantly slow or even prevent the disease,” Fillit said.
The blood test is the first of what researchers say could be a new generation of blood tests to replace expensive brain scans and spinal taps now used to diagnose Alzheimer’s, the most common form of dementia. Early diagnosis could allow patients earlier access to FDA-approved drugs to treat Alzheimer’s, an incurable disease that afflicts an estimated 7.2 million older Americans.
In a statement, FDA Commissioner Martin Makary said 10% of people aged 65 and older have Alzheimer’s. “I am hopeful that new medical products such as this one will help patients,” Makary said.
The Lumipulse blood test is made by Japanese company, Fujirebio Diagnostics, which markets a similar Alzheimer’s test that measures cerebrospinal fluid collected by spinal tap. The company did not return messages from USA TODAY asking when the blood test would be available and how much it will cost.
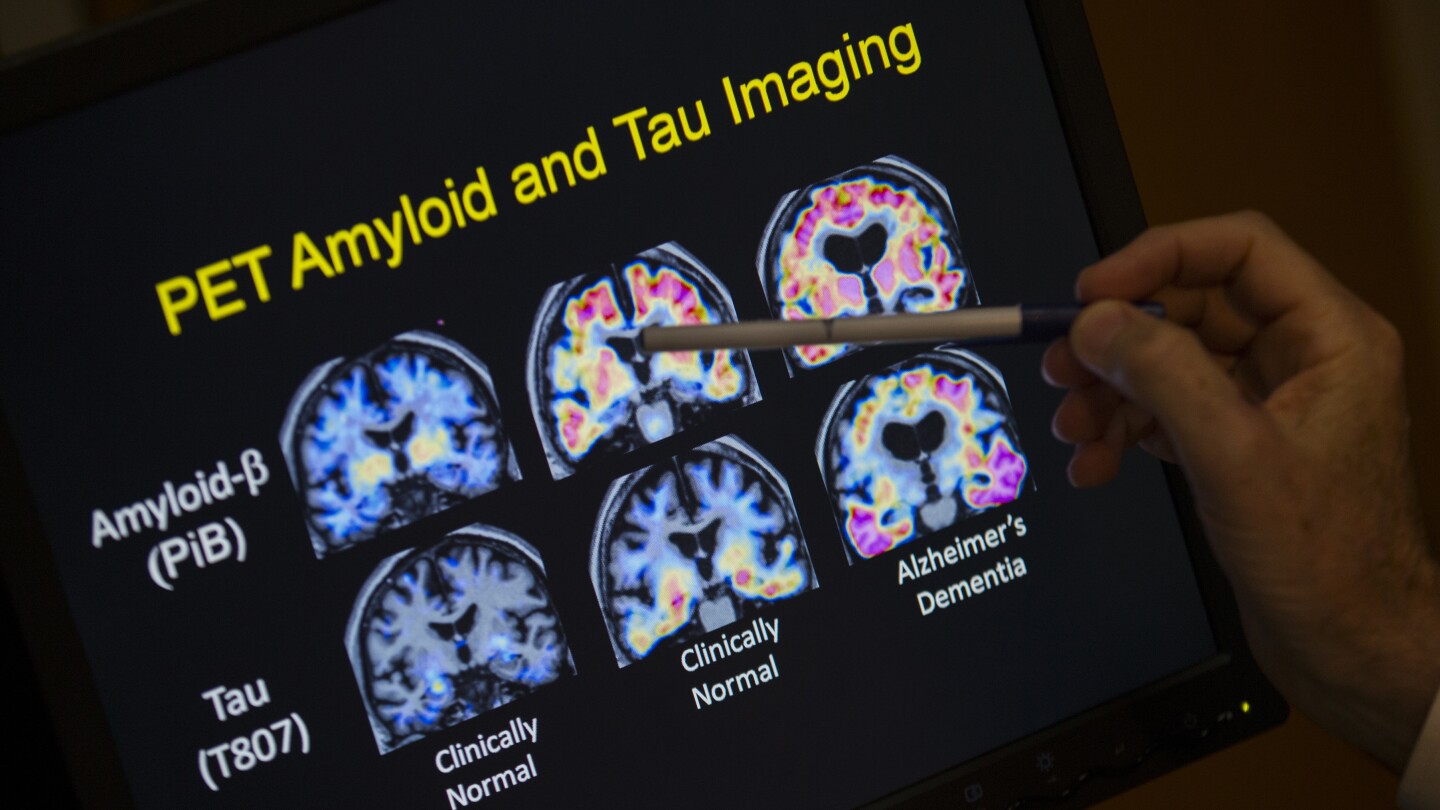
The test calculates a ratio of two proteins, tau and amyloid, found in blood plasma. The ratio approximates a measure of whether a patient has amyloid plaques in their brains − a disease hallmark that is now detected by images from expensive PET scans.
In clearing the new blood test, the FDA evaluated a clinical study that measured 499 samples from adults who were cognitively impaired. The study measured how often the blood test detected amyloid plaque compared to existing PET scans or spinal fluid tests.
The blood test picked up nearly 92% of cases detected by scans or spinal fluid. Less than 20% of cases received an inconclusive result, the FDA said.
In a news release, the FDA said the test can “reliably predict the presence or absence of amyloid pathology associated with Alzheimer’s disease” in people who are cognitively impaired. The test is meant to be used at memory clinics or other specialized care settings. Risks include possible false positive or false negative results.
Alzheimer’s researchers believe the disease takes root with brain changes before memory and thinking problems surface. The new generation of blood tests could lead to earlier diagnosis and give patients access to medication.
The FDA has approved two Alzheimer’s drugs targeting amyloid plaques in adults with early signs of the disease. Eli Lilly’s Kisunla and Eisai and Biogen’s drug Leqembi include warnings for MRI-visible injuries, which can include brain swelling and tiny bleeds at the surface of the brain.