The Food and Drug Administration approved on Friday the first blood test for diagnosing Alzheimer’s disease, opening up a quicker way for patients to get detected for the neurological condition and receive treatment.
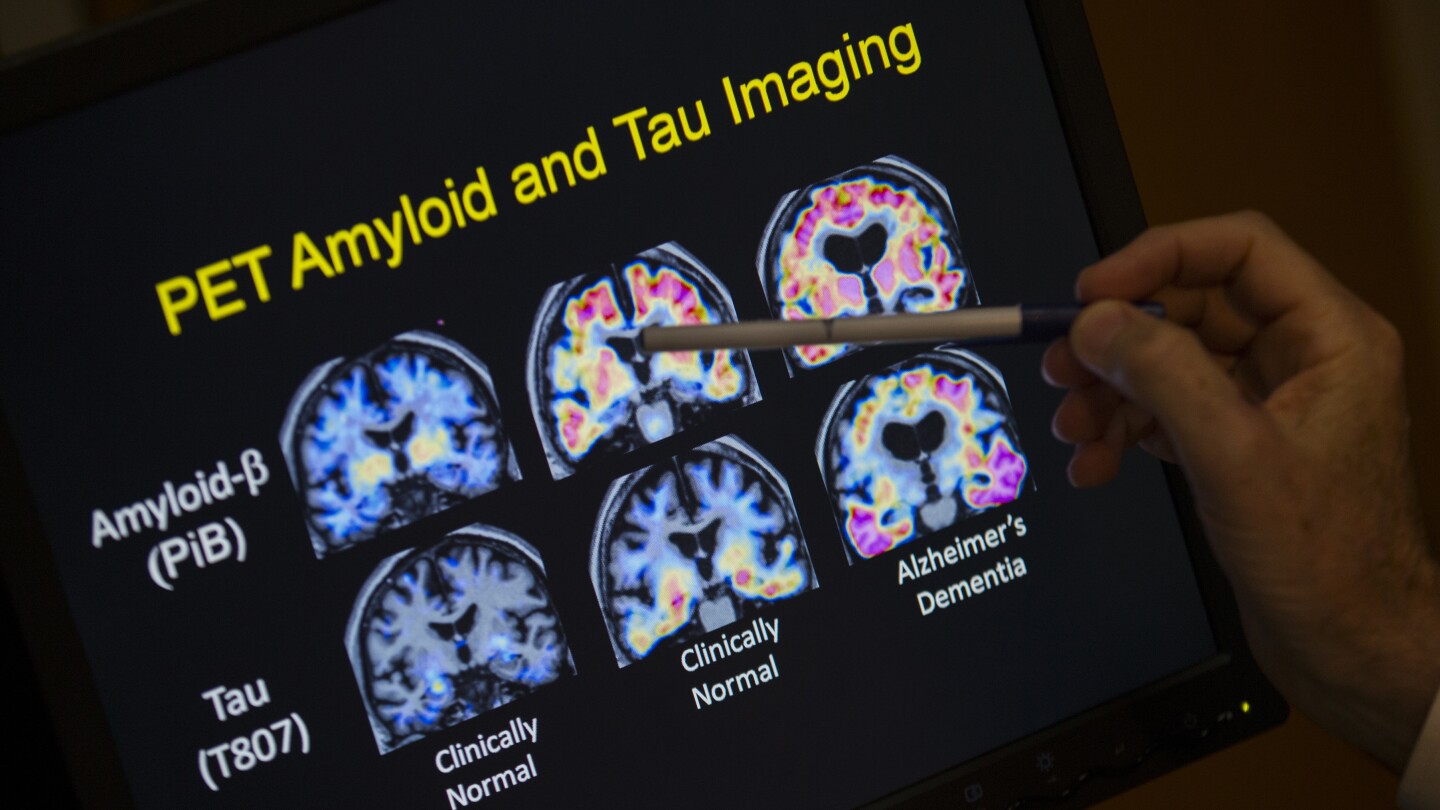
The test, developed by Japan-based Fujirebio Diagnostics, uses a blood sample to detect the presence of plaques in the brain called amyloid that are thought to be linked to Alzheimer’s. The current method of detecting amyloid involves PET imaging scans of the brain that can be inconvenient and costly. Amyloid can also be detected in spinal fluid, but such tests are more invasive.
“Nearly 7 million Americans are living with Alzheimer’s disease and this number is projected to rise to nearly 13 million,” said Michelle Tarver, director of the FDA’s Center for Devices and Radiological Health, in a statement. “Today’s clearance is an important step for Alzheimer’s disease diagnosis, making it easier and potentially more accessible for U.S. patients earlier in the disease.”
Two drugs to treat Alzheimer’s disease are currently approved in the U.S., but uptake has been limited, partly due to the logistical challenges of diagnosing the disease early enough for the drugs to be effective. Biogen and Eisai, the Japanese drugmaker, market the Alzheimer’s treatment Leqembi. Eli Lilly sells the other approved Alzheimer’s drug, called Kisunla.
“We welcome the FDA’s approval of the first blood-based test to support the evaluation of Alzheimer’s disease,” said Priya Singhal, Biogen’s head of development. “This represents a meaningful step forward in improving access to timely diagnosis, including in primary care settings where early signs of cognitive decline are often first observed.”
Similarly, in a statement, Lilly said the approval “marks a monumental breakthrough in Alzheimer’s disease diagnostics.”
Jason Karlawish, a professor of medicine at the University of Pennsylvania who specializes in Alzheimer’s research, said that “used right, this is a test that could really help to improve the diagnostic experience.”
But with the availability of easier-to-use tests, there’s always the risk of “some frisky prescribing habits,” Karlawish said. Particularly in the field of Alzheimer’s, where just a small number of doctors are trained to treat the increasingly common condition, “the outcome can be inappropriate prescribing of the tests because a lot of people have a desire to get it, but not a lot of clinicians know how to properly use it.”
The test should only be used to help diagnose people who have confirmed cognitive impairment, and there’s a risk some doctors may skip the step of confirming, as it’s “much easier to order a test than it is to talk to a patient,” Karlawish said.
Still, “in the history of Alzheimer’s disease, this is a big day,” he said. “The notion 10 years ago that there’d be a blood test that detects the pathologies of Alzheimer’s was a bit of a science fiction fantasy kind of story, and now here it is FDA-approved and ready for clinical practice.”
Lilly has been trying to make it easier for patients to get diagnosed, recently striking an agreement in which it connects patients to a telehealth company that can diagnose Alzheimer’s and prescribe treatments. This kind of partnership, though, is part of a broader direct-to-consumer push by pharma companies that experts worry may lead to overprescribing and inadequate care.
The newly cleared test from Fujirebio measures two proteins, pTau217 and beta-amyloid 1-42, found in human plasma, a component of blood. A numerical ratio of the levels of the two proteins is correlated to the presence or absence of amyloid plaques in the brain.
Other diagnostic companies have developed similar blood-based tests for Alzheimer’s, but the one from Fujirebio is the first to receive clearance from the FDA.
In a clinical study, 92% of individuals with positive blood-test results had the presence of amyloid plaques confirmed by either a PET scan or a spinal-fluid test, while 97% of individuals with negative results from the blood test also had confirmed negative results using the other methods, the FDA said.